 |
The shapes of \( \mathrm{XeF} {4}, \mathrm{XeF} {5}^{-} \)and \( \mathrm{SnCl} {2} \) are : \( \...
(PW Solutions)
View
|
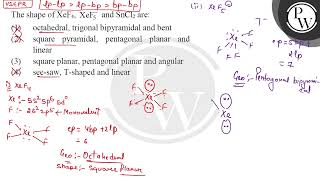 |
The shape of \( \mathrm{XeF} {4}, \mathrm{XeF} {5}^{-} \)and \( \mathrm{SnCl} {2} \) are....
(PW Solutions)
View
|
 |
The shapes of `XeF (4),XeF (5)^(-) and SnCl (2)` are :
(Doubtnut)
View
|
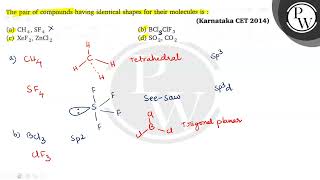 |
The pair of compounds having identical shapes for their molecules is : (a) \( \mathrm{CH} {4}, \...
(PW Solutions)
View
|
 |
In which one of the following species, the central atom has the type of hybridization which is n...
(PW Solutions)
View
|
 |
How many molecules or ions are linear in shape `{:(BeCl (2),XeF (2),CIF (2)^(-),I (3)^(-)),(I
(Doubtnut)
View
|
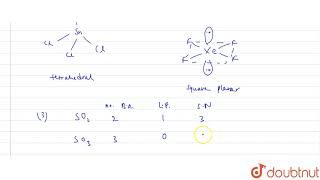 |
In which of the following pairs do the species have identical shapes
(Doubtnut)
View
|
 |
()
View
|
 |
()
View
|
 |
()
View
|
 Kamis, 20 Maret 2025 (04:34)
Kamis, 20 Maret 2025 (04:34)




